0710-4.DISCUSSION (07104电池能复活?)
0710-4.DISCUSSION (07104电池能复活?)
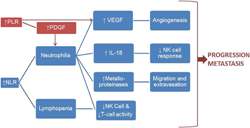
Figure 3 Rationale for predictive and prognostic role of NLR and PLR
Rationale for predictive and prognostic role of NLR and PLR4.1 Predictive immunotherapy biomarkers in HCC—an unmet need
Several Immunotherapy trials have demonstrated substantially higher response rates, greater tolerability, and a trend toward improved survival in favor of anti–PD‐1 therapy over sorafenib in HCC, driven by a subset of patients with clinically meaningful and durable responses.
Analyses of the genomic landscape of HCC including TMB, PD‐1, DDR alterations have so far been unable to establish predictive biomarkers of response to anti–PD‐1 therapy.7, 25-27
Historically, diagnosis of HCC based on characteristic radiological findings on dynamic contract imaging has been repeatedly validated and is highly sensitive and specific, taking away the need for tissue diagnosis.28
Furthermore, imaging eliminates the morbidity and mortality associated with the biopsy procedure as well as the concern for tumor seeding;
this however, also means there is often a lack of tumor tissue for molecular and microscopic biomarker analysis.
It is thus imperative to explore nontissue based biomarkers, such as serum biomarkers which are readily available, cost effective, and easy to interpret.
This study to our knowledge is the first to evaluate the role of NLR, PLR, and their combination as predictive biomarkers of survival at baseline as well as after three cycles of anti–PD‐1 therapy in aHCC.
It addresses the need for noninvasive biomarkers capable of stratifying aHCC patients based on their likelihood of responding to immunotherapy and for indicators of on‐treatment response.
4.2 Mechanistic rationale
The mechanistic rationale for the prognostic and predictive value of inflammatory markers in solid tumors has been well discussed in literature.14, 29, 30
Neutrophilia, as reflected in an elevated NLR, leads to increased production of neutrophil‐derived cytokines such as vascular endothelial growth factor (VEGF), matrix metalloproteinases, and interleukin‐18 (IL‐18).
VEGF promotes angiogenesis and metalloproteinases increase extravasation and inflammation.
IL‐18 impairs NK and T cell function, thus impairing host immune responses to tumor antigens.31
Thrombocytosis plays a similar role, mediated by VEGF and platelet‐derived growth factor (PDGF) production, which further recruits neutrophils and monocytes.32
These cascades of events thus promote tumor progression and metastasis (Figure 3).
以上就是(0710-4.DISCUSSION (07104电池能复活?))全部内容,收藏起来下次访问不迷路!